acid–base titrations
Difficulty Levels:
1
Q&A
Easy
Dilute sulfuric acid and aqueous sodium hydroxide are used to make aqueous sodium sulfate,$$\mathrm{Na}_{2} \mathrm{SO}_{4}(\mathrm{aq})$$,or aqueous sodium hydrogen sulfate,$$\mathrm{NaHSO}_{4}(\mathrm{aq})$$.The method includes use of the following apparatus.
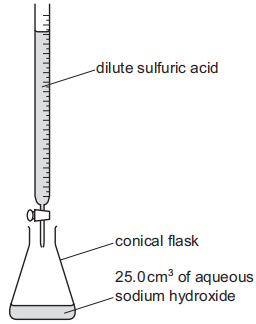 $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide of concentration $$0.100 \mathrm{~mol} / \mathrm{dm}^{3}$$ was neutralised by $$25.0 \mathrm{~cm}^{3}$$ of dilute sulfuric acid of concentration $$0.0500 \mathrm{~mol} / \mathrm{dm}^{3}$$.The equation for the reaction is shown.This is reaction $$1 .$$
$$
2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})\quad\quad reaction 1
$$
The same technique and the same solutions can be used to make aqueous sodium hydrogen sulfate.The equation for the reaction is shown.This is reaction $$2 .$$
$$
\mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{NaHSO}_{4}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})\quad\quad reaction 2
$$
Complete the table to calculate the volume of dilute sulfuric acid that reacts with $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide in reaction 2.
$$
\begin{array}{|l|c|c|}
\hline & \begin{array}{c}
\text { volume of } 0.0500 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { dilute sulfuric acid in } \mathrm{cm}^{3}
\end{array} & \begin{array}{c}
\text { volume of } 0.100 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { aqueous sodium hydroxide in } \mathrm{cm}^{3}
\end{array} \\
\hline \text { reaction 1 } & 25.0 & 25.0 \\
\hline \text { reaction 2 } & & 25.0 \\
\hline
\end{array}
$$
$$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide of concentration $$0.100 \mathrm{~mol} / \mathrm{dm}^{3}$$ was neutralised by $$25.0 \mathrm{~cm}^{3}$$ of dilute sulfuric acid of concentration $$0.0500 \mathrm{~mol} / \mathrm{dm}^{3}$$.The equation for the reaction is shown.This is reaction $$1 .$$
$$
2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})\quad\quad reaction 1
$$
The same technique and the same solutions can be used to make aqueous sodium hydrogen sulfate.The equation for the reaction is shown.This is reaction $$2 .$$
$$
\mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{NaHSO}_{4}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})\quad\quad reaction 2
$$
Complete the table to calculate the volume of dilute sulfuric acid that reacts with $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide in reaction 2.
$$
\begin{array}{|l|c|c|}
\hline & \begin{array}{c}
\text { volume of } 0.0500 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { dilute sulfuric acid in } \mathrm{cm}^{3}
\end{array} & \begin{array}{c}
\text { volume of } 0.100 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { aqueous sodium hydroxide in } \mathrm{cm}^{3}
\end{array} \\
\hline \text { reaction 1 } & 25.0 & 25.0 \\
\hline \text { reaction 2 } & & 25.0 \\
\hline
\end{array}
$$
 $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide of concentration $$0.100 \mathrm{~mol} / \mathrm{dm}^{3}$$ was neutralised by $$25.0 \mathrm{~cm}^{3}$$ of dilute sulfuric acid of concentration $$0.0500 \mathrm{~mol} / \mathrm{dm}^{3}$$.The equation for the reaction is shown.This is reaction $$1 .$$
$$
2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})\quad\quad reaction 1
$$
The same technique and the same solutions can be used to make aqueous sodium hydrogen sulfate.The equation for the reaction is shown.This is reaction $$2 .$$
$$
\mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{NaHSO}_{4}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})\quad\quad reaction 2
$$
Complete the table to calculate the volume of dilute sulfuric acid that reacts with $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide in reaction 2.
$$
\begin{array}{|l|c|c|}
\hline & \begin{array}{c}
\text { volume of } 0.0500 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { dilute sulfuric acid in } \mathrm{cm}^{3}
\end{array} & \begin{array}{c}
\text { volume of } 0.100 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { aqueous sodium hydroxide in } \mathrm{cm}^{3}
\end{array} \\
\hline \text { reaction 1 } & 25.0 & 25.0 \\
\hline \text { reaction 2 } & & 25.0 \\
\hline
\end{array}
$$
$$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide of concentration $$0.100 \mathrm{~mol} / \mathrm{dm}^{3}$$ was neutralised by $$25.0 \mathrm{~cm}^{3}$$ of dilute sulfuric acid of concentration $$0.0500 \mathrm{~mol} / \mathrm{dm}^{3}$$.The equation for the reaction is shown.This is reaction $$1 .$$
$$
2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{Na}_{2} \mathrm{SO}_{4}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})\quad\quad reaction 1
$$
The same technique and the same solutions can be used to make aqueous sodium hydrogen sulfate.The equation for the reaction is shown.This is reaction $$2 .$$
$$
\mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{4}(\mathrm{aq}) \rightarrow \mathrm{NaHSO}_{4}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})\quad\quad reaction 2
$$
Complete the table to calculate the volume of dilute sulfuric acid that reacts with $$25.0 \mathrm{~cm}^{3}$$ of aqueous sodium hydroxide in reaction 2.
$$
\begin{array}{|l|c|c|}
\hline & \begin{array}{c}
\text { volume of } 0.0500 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { dilute sulfuric acid in } \mathrm{cm}^{3}
\end{array} & \begin{array}{c}
\text { volume of } 0.100 \mathrm{~mol} / \mathrm{dm}^{3} \\
\text { aqueous sodium hydroxide in } \mathrm{cm}^{3}
\end{array} \\
\hline \text { reaction 1 } & 25.0 & 25.0 \\
\hline \text { reaction 2 } & & 25.0 \\
\hline
\end{array}
$$
Answer:
$$50.0\left(\mathrm{~cm}^{3}\right)$$
Explanation:








